What is GaN
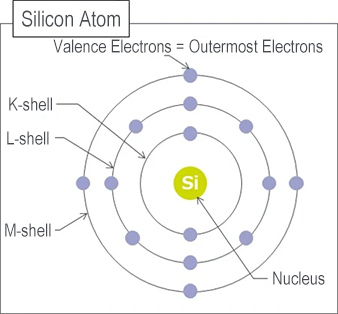
GaN (Gallium Nitride) is a compound semiconductor composed of Gallium (Ga) and Nitrogen (N).
A compound semiconductor is a type of semiconductor consisting of two or more elements bonded together.
Other compound semiconductors include SiC (Silicon Carbide), GaAs (Gallium Arsenide), and InAs (Indium Arsenide).
GaN provides superior physical properties compared to Si, including high temperature tolerance, high speed operation, critical operating voltage, and low power consumption.
At the same time, GaN and SiC are classified as wide bandgap semiconductors due to their larger bandgaps than Si. A wider bandgap translates to a higher potential breakdown voltage.
Click here fore a comparison table of semiconductor physical properties >
Electron Mobility (cm2/Vs): GaN (2DEG=2 Dimensional Electron Gas) 1500~2001
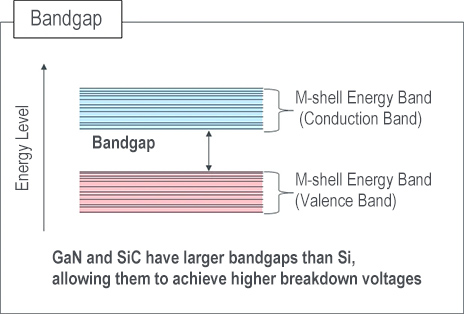
Bandgap
The energy that an electron can possess corresponds to specific values associated with energy levels known as electron orbitals (s, p orbital). This range of energy levels forms what's known as an energy band.
More specifically, the energy band characterized by higher energy levels is termed the conduction band, while the energy band with lower energy levels is referred to as the valence band.
In addition, sandwiched between energy bands is a range where electrons cannot exist, known as the forbidden band, where the energy difference between the upper and lower boundaries of the forbidden band is called the bandgap.
In other words, the bandgap is the energy required for an electron to transition from the valence band to the conduction band.
Si has a bandgap of 1.12eV, SiC a bandgap of 3.26eV, and GaN a bandgap of 3.5eV.
The unit for bandgap is eV (electron volt), which is defined as ‘the energy gained by one electron when accelerated by a voltage of 1V, where 1eV is approximately equal to 1.602×10-19J.
As the temperature rises, a phenomenon can occur where electrons transition from the valence band to the conduction band due to thermal energy, leading to unintended behavior.
For electrons to transition, wide bandgap semiconductors like GaN and SiC require more thermal energy than Si. This allows SiC and GaN to achieve higher breakdown voltages.