Battery Types
Primary and Secondary Batteries
Batteries can broadly be divided into primary "and secondary batteries.
A primary battery is a ‘disposable’ type that is thrown away once it is completely used up.
In contrast, a battery that can be repeatedly recharged is called a secondary or rechargeable battery.
- Primary Batteries (Disposable)
●Alkaline batteries
●Manganese batteries
●Mercury batteries (button)

- Secondary Batteries (Rechargeable)
●Nickel-Metal Hydride Batteries
●Nickel-Cadmium Batteries
●Lead-Acid Batteries
●Li-ion Batteries
●Fully-/Semi-Fixed Batteries

■Li-ion Batteries
The most common type of rechargeable battery is the li-ion type.
The positive electrode is composed of li-ion metal oxide and the negative electrode made up of carbon, with an electrolyte filling in between.
It is often used in compact and mobile electronic devices due to its small size, light weight, high energy density, and easy access to high voltages.
- Advantages
-
- Higher capacity and output vs nickel-metal and lead-acid batteries
- Actual capacity does not decrease through repeated charging
- Drawbacks
-
- Charging outside the recommended range of 0°C to 45°C※ is not recommended
- Risk of ignition at high temperatures with rapid deterioration of characteristics at low temperatures
※Charging should be performed within the temperature conditions specified by the manufacturer
■All-/Semi-Solid State Batteries
The increasing demand for safer, higher density rechargeable batteries as devices have become smaller and thinner has led to the development and commercialization of new battery types, including all-solid/semi-solid models using novel materials for the electrolytic part.
Unlike li-ion batteries that use an electrolyte, all-solid state batteries feature a solid body that eliminates the risk of leakage.
What's more, there is no need to shield the electrode and electrolyte, and the lower battery voltage of 2-3V vs the 3-5V of li-ion types translates to higher efficiency.
●All-Solid State Batteries
(Utilizes a solid instead of an electrolyte)
●Semi-Solid Batteries
(Utilizes an organic electrolyte as a electric field solution)
Click here for ROHM’s lineup of charge control ICs that support low voltage charging
- Advantages
-
- Capable of charging at a current greater than the nominal battery capacity of 1C※
- Wide operating temperature range (-40°C to 105°C recommended for some products)
- Drawbacks
-
- Lower energy density relative to volume vs li-ion, so capacity cannot be gained
※C Rate: Defines 1C as the amount of current required to fully charge and discharge the nominal battery capacity in one hour
Key Battery Terms
| Terminology | Definition |
|---|---|
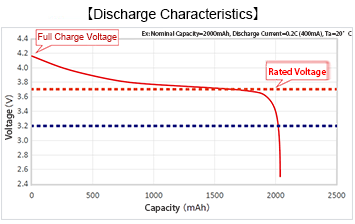
| Rated Voltage | The voltage that can be output for the longest time on average *This is 3.7V for typical li-ion batteries |
| Full Charge Voltage | Voltage at full charge. Basically this is above the rated voltage *This is 4.2V for typical li-ion batteries *Technological innovations in recent years have made 4.35V and 4.4V available *Some all-solid state batteries can be as low as 2.8V |
| Battery Capacity Unit (mAh) |
Unit for battery capacity(mAh) Defines 1C as the current value at which the capacitance becomes zero after 1 hour of discharge *The typical charging current is less than 1C *Charging performed from 0.5 to 0.7C, as charging above this level can cause ignition *Some batteries can be charged at 1C or higher due to technological innovations in charging current |
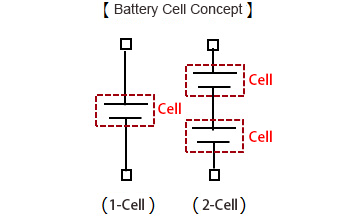
| No. of Cells | Higher output voltages are achieved by stacking individual 4.2V cells, resulting in 1-cell (1S), 2-cell (2S), etc. configurations. *Cells can be connected in parallel to increase capacity 2S2P (2-cell, 2-para), etc. |
Battery Charge ICDownload datasheet
The next page briefly explains the charging method of a charge control IC